Gallium is a rare metal that melts in your hands
Categories: Science
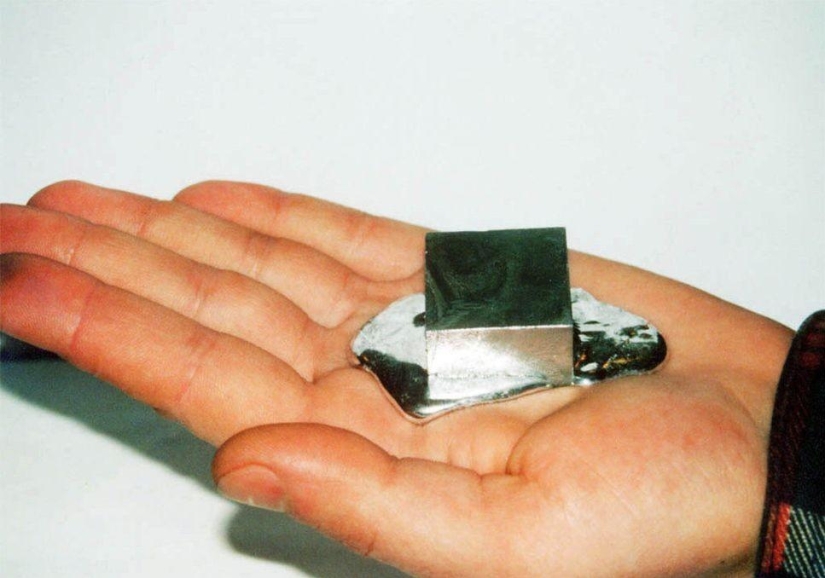
By Pictolic https://pictolic.com/article/gallium-is-a-rare-metal-that-melts-in-your-hands.htmlGallium is a metal whose melting point is only 30 °C, i.e. it melts from the warmth of hands. At the same time, it is harmless to humans and can be used as a fun toy.

Gallium is a chemical element with atomic number 31. It belongs to the group of light metals and is indicated by the symbol "Ga". Gallium in its pure form does not occur in nature, but its compounds are found in negligible amounts in bauxite and zinc ores.

Gallium is a soft plastic metal of silver color. At low temperatures, it is in a solid state, but melts already at a temperature not much higher than room temperature (29.8 ° C). In the video below, you can see how a gallium spoon melts in a cup of hot tea.

From the discovery of the element in 1875 until the advent of the semiconductor era, gallium was mainly used to create fusible alloys.

Currently, all gallium is used in microelectronics. Gallium arsenide, the main element compound used, is used in microwave circuits and infrared optics.
Gallium nitride is used less when creating semiconductor lasers and LEDs in the blue and ultraviolet range.

Gallium has no biological role known to science. But, since gallium compounds and iron salts behave similarly in biological systems, gallium ions often replace iron ions in medical applications.

Galium is non-toxic to humans, provided that it is not swallowed and vapors are not inhaled. Molten metal leaves grayish marks on the hands, but it is easy to get rid of them by washing your hands with soap and water.
Recent articles

It's high time to admit that this whole hipster idea has gone too far. The concept has become so popular that even restaurants have ...

There is a perception that people only use 10% of their brain potential. But the heroes of our review, apparently, found a way to ...

New Year's is a time to surprise and delight loved ones not only with gifts but also with a unique presentation of the holiday ...